
International pharmaceutical company

• NOVAST was awarded Best Supplier of the Year 2021 by Healthcare UC (Mckesson).
• Metformin Hydrochloride Sustained-release Tablets (III) and Metoprolol Succinate ER Tablets were approved by China NMPA.

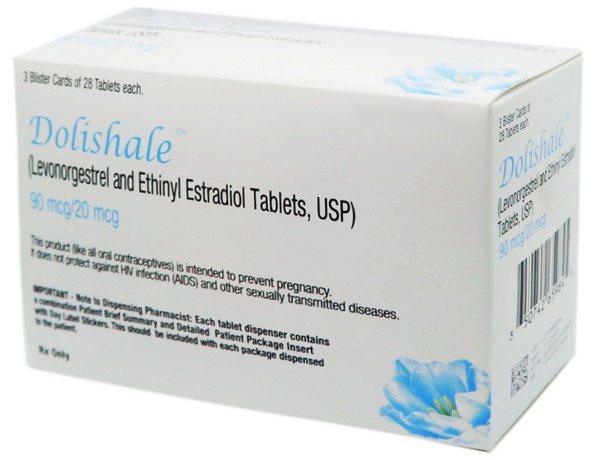
DOLISHALE™, the 40th Novast product is approved by the US FDA.
• NOVAST was awarded Best Supplier of the Year 2018 by NorthStar Healthcare UC (Mckesson).
• NOVAST was awarded Best Business Partner of the Year 2019 by Ingenus.

• NOVAST completed the extension project of the 25,000 square meter CGMP manufacturing suite.
• NOVAST has completed successful 5 consecutive FDA inspections with no 483 observation from 2013 to 2018.

Nifedipine ER Tablets, the First Novast Extended-Release Product is launched into the US Market.

By 2015, 20 products developed by NOVAST had been approved by US FDA.
NOVAST was designated as a New High-tech Enterprise by People's Republic of China.

PHILITH™, the FIRST NOVAST oral contraceptive product successfully launched into the US mainstream oral contraceptive prescription drug market.

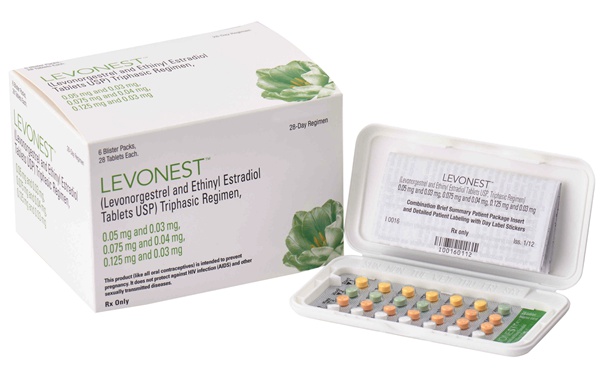
LEVONEST™, the FIRST NOVAST Product Approved by the US FDA.
NOVAST submitted our FIRST ANDA applications to the US FDA and received a successful CGMP on-site inspection by the US FDA.

